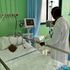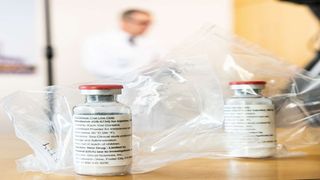
This file photograph taken on April 8, 2020, shows vials of the drug 'remdesivir' during a press conference on Covid-19 medication at The University Hospital Eppendorf (UKE) in Hamburg, northern Germany.
| Ulrich Perrey | AFPNews
Premium
Four foreign firms apply to supply Kenya with Covid-19 drug
Kenya has received four applications from foreign pharmaceuticals seeking to import a drug cleared to treat severe Covid-19 patients.
The Pharmacy and Poisons Board (PPB) disclosed Square Pharmaceuticals (Bangladesh), Beximco Pharmaceuticals (Bangladesh) and Cipla Ltd (India) have applied to supply the country with Remdesivir, an antiviral drug.
Gilead Life Sciences, the manufacturer of Veklury (the innovator for Remdesivir) has also expressed interest to supply the drug through the Ministry of Foreign Affairs.
Full approval
It is one of the five companies licenced to manufacture the drug for developing countries.
This comes after the US Food and Drug Administration (FDA) granted full approval to the antiviral drug for treatment of Covid-19 patients.
In October, a study by the World Health Organization (WHO) found that Remdesivir, along with three other drugs, had little or no effect despite the drug being used to treat President Donald Trump after he tested positive for coronavirus.
However, the European Commission was first to give conditional approval for the use of Remdesivir in severe Covid-19 patients following an accelerated review. The drug, first developed to treat Ebola, was, however, approved for emergency treatment of Covid-19.
This was done immediately after conditional authorisation was given in May 2020 considering that Kenya was on the list to receive the vaccine which is also being used and sold in India.
PPB told the Nation that there has been a pre-submission meeting by one of the multinationals for an application for emergency use authorisation of Covid-19 vaccine.
Proven efficacy
The applicants stated that they have proven efficacy and safety but the board is still awaiting submission of data as evidence.
“The advantage is that the vaccine is based on a vaccine platform that has been used previously which assures better safety,” said an official who preferred the information is attributed to the board.
Remdesivir
The Nation had reported in July that Kenya is among 126 low-income and lower-middle-income countries identified by the big pharmaceutical to receive the drug.
“We are yet to receive their application for issuance of emergency use authorisation or full registration,” the board said.
The board explained that all the applications were still under review and further compassionate use authorization has been issued from time-to-time for needy patients. Once the applications are successful it would allow import and distribution.
Donations
The board also revealed that Kenya has not depleted the consignment of drugs donated by India for HIV and Covid-19 through the Ministry of Foreign Affairs.
The donation contained several medicines including metronidazole, levocetrizine, montelukast, ceftriaxone, hydroxychloroquine and chloroquine.
This comes after Kenya this week asked the international community to promote equitable access to Covid-19 vaccine amid the latest breakthrough at the late-stage trials conducted by global manufacturers.
“Covid-19 vaccines are a common public good for health that should be accessed by countries in a fair manner,” Patrick Amoth, Kenya’s director-general in the Ministry of Health told a virtual WHO assembly.
Kenya had purchased more Hydroxychloroquine from India in May joining an exclusive list of countries that opted to ‘repurpose’ the banned antimalarial drug.
“In keeping with excellent bilateral ties and as a special gesture, India has allowed one-time export of prohibited Hydroxychloroquine Sulphate USP 200 mg (379,000 tablets) to Kenya to support Government of Kenya in its fight against Covid-19 pandemic,” read a statement from the Indian government.
Hydroxychloroquine is mainly used for treatment of arthritis though clinical studies and case reports done so far indicate that the drug has not shown any efficacy in treatment of covid-19.
Challenges
The availability of effective rapid testing kits which are easy to use and cost effective is the biggest challenge the board says it is facing at the moment.
“We believe it is possible to get us rapid test kits (antigen and antibody based) that are highly specific and highly sensitive.”
Currently, the Ministry of Health has settled on PCR based kits which are used for confirmatory tests as their testing kits of choice.
According to WHO, Covid-19 Coronavirus Real Time PCR Kit is an In Vitro Diagnostic (IVD) reagent replying on fluorescent PCR technology and aiming at qualitatively detecting SARS-CoV-2 from upper and lower respiratory tract specimens.
Upper respiratory tract specimens include throat swab and nasopharyngeal swab. Lower respiratory tract specimens include sputum.
The other challenge is a deluge of applications outstretching PPB’s human resource capacity.
However, most employees are well equipped to work at home using the online system , Pharmaceutical Regulatory System (PRIMS) which helps limit personal interaction between staff and clients thus controlling the spread of Covid-19 .
Treatment
The pharmacy and poisons board also reveals there is what it calls ‘investigational medicine’ that will be available in the near future such as monoclonal antibodies on experimental basis.
“We have also received an antiviral Favipiravir (Fabiflu tablets from Glenmark Pharmaceuticals Ltd) which is now available across the country for compassionate use.”
On November 9th 2020, the FDA issued an emergency use authorisation for the investigational monoclonal antibody therapy, bamlanivimab, which is used to treat mild-to-moderate COVID-19 in adult and paediatric patients.
Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to fight off harmful antigens such as viruses.
The FDA also says that Bamlanivimab is authorised for patients with positive results of direct SARS-CoV-2 viral testing who are aged 12 years and older weighing at least 40 kilograms, and who are at high risk of progressing to severe Covid-19 and or hospitalisation.
They include those aged 65 or older, or who have certain chronic medical conditions.
Bamlanivimab is specifically directed against the spike protein of SARS-CoV-2, designed to block the virus’ attachment and entry into human cells.
The FDA however noted that monoclonal antibodies such as bamlanivimab, may be associated with worse clinical outcomes when administered to hospitalized patients with Covid-19 requiring high flow oxygen or mechanical ventilation.