Poisons board stops sale of cough syrup after death claims
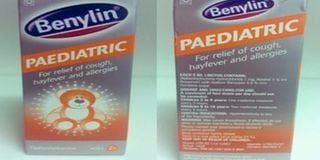
Benylin Paediatric cough syrup which has been recalled by the Pharmacy and Poisons Board.
What you need to know:
- Benylin Pediatrics is also used for treatment of hay fever and allergies in children.
- Other variations available for sale in Kenya include the Dry Cough and Original.
Hundreds of thousands of Kenyan children could have been exposed to the harmful effects of a popular cough syrup that has been recalled in other countries after it was linked to several deaths of minors.
The Pharmacy and Poisons Board issued a warning this evening against the distribution, sale and use of a particular batch Benyline Pediatrics cough syrup, but fell short of ordering a total recall.
Benylin Pediatrics is also used for the treatment of hay fever and allergies in children aged six years and above.
Nation.Africa understands that the version of the syrup on sale locally and the one banned in Nigeria are both made in South Africa by pharmaceutical giant Johnsons & Johnsons.
Ordering the stop of the sale of the affected batch of the drug, Dr Fred Siyoi, chief executive officer of the Pharmacy and Poisons Board, advised members of the public to return the product to the nearest healthcare facility.
The mop-up will extend from the pharmacies to their suppliers, but will only relate to batch number 329304 of the 100mls syrup.
A spot check by Nation.Africa this evening established that the product was still on sale in some physical and online outlets.
Besides the Pediatrics syrup, other common variations available for sale in Kenya include the Dry Cough and Original offers of the Benylin range.
Nigeria’s National Agency for Food and Drugs Administration and Control (NAFDAC) on Wednesday said that laboratory tests on Benylin Paediatric showed a high level of diethylene glycol, which were also found in cough syrups that have killed dozens of children in Gambia, Uzbekistan and Cameroon since 2022.
The agency added that the medicine was also found to cause acute oral toxicity in laboratory animals.
The particular lot recalled in Nigeria is set to expire this month, the regulator said, asking those in possession of the product to immediately discontinue sale or use.
The authorities urged anyone who has taken the medication to be aware of potential side effects, which include intense abdominal pain, vomiting, and diarrhoea, along with difficulty urinating.
Headaches, confusion, and signs of potential kidney damage, such as fatigue, swelling, or a metallic taste, should also be taken seriously.
According to the World Health Organisation (WHO), Diethylene glycol is an alcoholic toxic chemical used in industrial applications such as the manufacture of paints, ink or brake fluids.