Oxford Covid-19 vaccine trials on
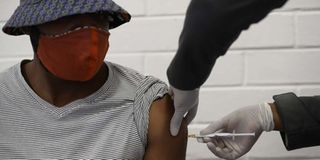
One of the first South African Oxford vaccine trialists looks on as a medical worker injects him with the clinical trial for a potential vaccine against the Covid-19 coronavirus at the Baragwanath hospital in Soweto, South Africa, on June 24 ,2020.
Everything in the Covid-19 vaccine trials in Kenya is “going on as expected”, the scientists running the study told the Sunday Nation, even as the Health minister said the team deserves “real credit”.
A few days after coming under attack from the scientific community for his comments on vaccines, Health Cabinet secretary Mutahi Kagwe said the team in Kilifi was conducting “critical work.”
Mr Kagwe reiterated his comments that the ministry would not commit to just one study, including the vaccine at Kilifi.
“We will explore all options,” he wrote to the Sunday Nation.
Prof George Warimwe, the principal investigator running the study in Kenya, said the team at the research centre based in Kilifi relied on the existing knowledge and infrastructure for major research to run the Covid-19 study.
Prof Warimwe assured the public that while Covid-19 is a new virus that the scientific community is coming to terms with, the centre in Kilifi has carried out trials for other deadly viruses. The Wellcome Trust programme in Kilifi carried out the studies that proved the current Ebola vaccine rVSV ZEBOV is safe, alongside other research centres in Switzerland, Germany and Gabon.
The ChAdOx1 nCov-2019 Oxford coronavirus vaccine trials began on October 28 and will recruit 40 frontline workers in Kilifi. Once the safety is confirmed, the study will recruit another 360 and expand the research to 360.
The 400 would join another 20,000 volunteers taking part in the same vaccine trials in the United Kingdom, South Africa and Brazil. The vaccine is tried on frontline healthcare workers who are more at risk.
However, the researchers test them, and if they are found to have been infected with the virus, they are ineligible to participate in the trial.
Diverted resources
A vaccine will be a game-changer against Covid-19, which has since infected more than 68,000 and claimed nearly 1,300 lives in Kenya. The virus has also strained the health system and diverted some of the scarce resources such as workers and money solely to the management and care of Covid-19, leaving other patients and diseases unattended.
Prof Warimwe did not disclose when he expects the vaccine to be ready for the public. However, the main researcher of the entire study, Prof Andrew Pollard, told UK-based publication The Guardian last week that there is a small chance of vaccinating the people by the end of this year, by Christmas. “I think there is a small chance of that being possible, but I just don’t know,’’ said Prof Pollard, who is based in the UK.
It was not clear what delayed the start of the Kenya arm of the Oxford vaccine, but it is possible that the regulatory process was slower than anticipated.
The work on the vaccine began in January. There is also the possibility of the politicisation of vaccines in Kenya, and resistance from the public who felt, albeit erroneously, that they would be used as guinea pigs.
Prof Warimwe said he and his team are working hard to assure the public that the study would not deliberately put them in harm’s way and appealed for opinion leaders to help them in this cause.
Viral vector vaccine
“It would be useless if the vaccines are ready, and they cannot be used on the public because they fear,” Prof Warimwe told the Sunday Nation.
Kenya and the Oxford team is working with pharmaceutical firm AstraZeneca is making the vaccine from a viral vector vaccine made from a weakened version of a common cold virus that causes infections in Chimpanzees.
The researchers have modified the virus to include the spike in the virus that causes Covid-19, SARS-Cov2; this spike is what the virus uses to attach itself to the lungs.
On October 26, the researchers also published some preliminary data in the journal Lancet which shows that the vaccine produces an immune response in older adults above 65, as well as the young.
The results came from a phase 2 trial, where hundreds of people are tested for safety. The study also demonstrated little reactogenicity, the scientific name for the side effects of a virus or medicine.
[email protected]. @VerahOkeyo





