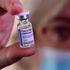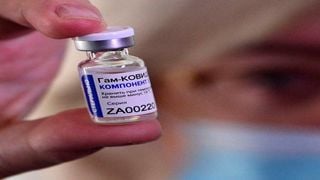
A medical worker displays a vial with Sputnik V vaccine during the vaccination of medics at a clinic in the far eastern city of Vladivostok on December 15, 2020.
| AFPNews
Premium
Revealed: Faces behind importation of Russian Covid-19 vaccine to Kenya
What you need to know:
- The Russian vaccine has a 92 per cent efficacy rate.
- Sputnik V is Kenya’s first private vaccine consignment.
The company approved to ship in and sell Covid-19 vaccines from Russia at a hefty price is owned by individuals who have benefited from some of the most lucrative medical deals in Kenya’s recent history, the Nation can reveal.
On Wednesday, the Pharmacy and Poisons Board (PPB) announced it had granted Emergency Use Approval to a private pharmacy in Kenya for the Russian-made Covid-19 vaccine, Sputnik V.
The vaccine, which has a 92 per cent efficacy rate, is Kenya’s first private vaccine consignment and can be received by those willing to cough up Sh8,000 per shot, according to various reports.
According to the certificate of approval awarded by the PPB, the deal was given to Dinlas Pharma EPZ Limited.
Reports indicate that at least 75,000 doses of the vaccine had arrived at the port of Mombasa by Sunday, a whole three days before it was announced that those who could afford it were at liberty to get vaccinated.
Dinlas Pharma EPZ was registered on January 26, 2018, according to investigations by the Nation.
The company was among the suppliers mentioned with regard to the Kenya Medical Supplies Authority’s (KEMSA) Covid-19 consignments of last year. The matter is currently being investigated by Parliament.
A search by the Nation’s Investigation desk revealed that Dinlas Pharma EPZ Limited is owned by Medillon Trading FZE and Parkdale Investments Limited, which have 341,700 and 168, 300 shares respectively.
Dubai-based companies
Medillon Trading FZE and Parkdale Investments Limited are both registered in Dubai in the United Arab Emirates.
While the Nation could not immediately ascertain the ownership of these two Dubai-based companies with shareholding in Dinlas Pharma EPZ, it is their Kenyan connection that seems to hold the key to the identities of the people behind the idea of vaccinating Kenyans at a cost.
Listed as a secretary of Dinlas Pharma EPZ is Ms Lenah Chelangat, while Mr Jayesh Umesh Saini is listed as a director of the company, but with no shares. Both are associated with Bliss GVS Healthcare Limited, a chain of hospitals that has benefited immensely from government medical schemes since its inception in 2012.
On her LinkedIn account, Ms Chelangat, who hails from Sotik in Bomet County, identifies herself as the company secretary for Bliss. She has also appeared a number of times on the company’s social media pages.
Mr Jayesh is the founder and chairman of Bliss. At one time he was mentioned adversely in the Clinix Healthcare scandal, where some Sh96 million was said to have been lost through a dubious medical scheme for civil servants and members of the disciplined forces.
Mr Jayesh was the chairman of Clinix at the time of the scandal.
Since its conception in 2012, Bliss has grown to more than 80 clinics spread across 45 counties in the country, making it one of the largest and fastest-growing networks of healthcare providers in the region.
Emergency use of Sputnik V
“We have more than 500 healthcare professionals working throughout our clinics and we serve more than 80,000 patients per month. With an extraordinary array of resources for the provision of compassionate, state-of-the-art care, Bliss Medical Centre is poised to identify and respond to the health-related needs of the diverse population we serve,” says the company on its website.
Bliss has also bagged several government contracts. In 2015, it won an annual service contract in relation to medical healthcare cover equivalent to $35 million (about Sh3.5 billion) in association with Aon Kenya Insurance Brokers.
The contract was supposed to provide outpatient medical services for more than 288,000 public school tutors under the Teachers Service Commission. The cover, which was supposed to be managed by AON, was to benefit the principal contributor, their spouse and four children.
The World Health Organization (WHO) has not yet authorised emergency use of Sputnik V as a vaccine, even though it is already in use in multiple countries across the globe. This begs the question why the distributor was so much in a hurry to have the vaccine in the market before distribution approval was officially obtained.
“This vaccine has so many forces behind it. This is not about the safety of the consignment but the politics behind it. If a consignment has been approved by a government entity, why would ministry officials say they are not aware, what are they hiding?” he posed.
The Head of Immunisation at the Health ministry, Dr Collins Tabu, on Wednesday said he was not aware of the Sputnik V vaccine’s existence in the country, even as the PPB said it had only approved its importation but not distribution.
Dr Willis Akhwale, the vaccine advisory task force chairman, said he was not privy to any discussions with anyone that arrived at such a decision at the Health ministry.
“The CEO has been directed to publish requirements of those who want to distribute the vaccines,” he said.
Conditions for distributor
Mr Jayesh started his career in the family-owned Nairobi West Hospital, and has since invested greatly in healthcare. Besides Bliss, he owns Medicross Ltd, comprising 22 outpatient centres equipped to offer comprehensive quality services.
Apart from the medical field, Mr Jayesh also has investments in the Energy sector through Surge Energy, which manufactures gas cylinders under the affordable clean-energy plan to eliminate charcoal, firewood and kerosene.
The Nation has learnt that one of the conditions that the distributor has to meet as per the Ministry of Health protocols before distribution, is to train nurses who are going to give the jab.
“When we brought the AstraZenecca vaccine, we trained healthcare workers, they also have to train before the actual rollout is done,” said an official.
Other conditions include ensuring user safety and registering users on the government-run Chanjo site, where everyone getting vaccinated have their details filed to enable the ministry to track the number of people taking the vaccine and any side effects.
The distributor also has to sign a technical agreement stipulating the responsibilities of all the parties involved in its administration.
The government signed an agreement with WHO waiving the right to sue any pharmaceutical company for any adverse effects of the AstraZeneca vaccine, which is currently the only Ministry of Heath-registered Covid-19 immunisation plan in the country.
Yesterday, Mr Nishant Mishra, a senior official at Dinlas Pharmaceuticals, said the vaccine has been imported with the full knowledge and clearance of the PPB.
Little risk of allergies
“We used Freight in Time Ltd as our clearing agent. We are using the Freight in Time’s cold room to (store it) at the required temperature levels. We have a tracker to monitor the temperature round the clock,” Mr Mishra told the Nation.
He denied allegations that the firm has made plans to release the jab, besides insisting that all their approvals were procedural.
“We obtained an Emergency Use Authorisation from the Pharmacy and Poisons Board on March 9 and we also have all other documents, licences and batch releases.
“The vaccine will be distributed through the health facilities’ networks. Freight in Time Ltd will help us do the distribution because they have the facilities to transport the vaccine at required temperatures.
“We will also be ensuring that the health facilities that receive the vaccine have cold-storage facilities,” said Mr Mishra on Wednesday.
The storage temperature recommended for Sputnik V is 2 to 8 degrees Celsius, which means it can be stored in a conventional refrigerator without the need for special cold-chain infrastructure.
The jab is also said to pose little risk of allergies. The two doses of the Russian vaccine are given 21 days apart, unlike the eight weeks apart for the Oxford AstraZeneca vaccine.
The AstraZeneca vaccine is already in use in the country after the delivery of 1.2 million doses under the Covax facility. About 56,000 people have been vaccinated countrywide so far.